
Rectal and esophageal gastrointestinal stromal tumors—how to deal with it?—technical approach and role of neoadjuvant treatment
Background
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal (GI) tract. GISTs likely originate from the interstitial cells of Cajal (ICCs), sometimes referred to as the GI pacemaker cells.
With an incidence range from 7 to 20 cases per million (1-3), these tumors can originate anywhere in the GI tract, however they are found predominantly in the stomach (60%), jejunum and ileum (30%). Less frequently, they are located in duodenum (5%), rectum (4–5%), colon and cecal appendix (1–2%).
Esophageal GISTs are very rare tumors, representing only 0.7% of all GISTs (4) with a low incidence of approximately 0.1 to 0.3 cases per million (5). Esophageal GISTs are typically diagnosed in patients older than 60 years and more frequently in men (6).
The data published so far for esophageal GISTs are scarce. Due to its low incidence, there are only individual case reports or series with few cases included. As a result of the above, there are currently no clear recommendations on the optimal management of this type of sarcoma (7,8).
Colorectal GISTs are rare and account for less than 5% of all these tumors, most of them being rectal (4). Its biological characteristics do not differ from those of other parts of the digestive tract (9), but they are more aggressive (10).
These two locations have certain anatomical characteristics that require specific management. Surgical resection is the only curative treatment, but it can be very aggressive. The role of neoadjuvant therapy is greater in these locations, and it can help to perform less aggressive and safer surgeries.
Esophageal GIST
Depending on the size and location of esophageal GISTs, the diagnosis may be made as an incidental finding in an asymptomatic patient, or a variety of signs and symptoms such as thoracic or abdominal pain/discomfort, dysphagia or esophageal obstruction and GI bleeding. For all the above, esophageal GISTs can lead to a variety of clinical presentations and treatment scenarios, where surgery plays a primary role (11).
The distinction between GISTs and leiomyomas is essential, as esophageal GISTs tend to pursue an aggressive clinical course, whereas leiomyomas are considered to be benign (3). The established treatment for localized esophageal or gastroesophageal junction GISTs is surgery. The two procedures that are considered are enucleation or esophagectomy (12).
Due to the special anatomy and the vascularization of the esophagus, wedge resections are not usually performed. Thus, enucleation or esophagectomy are usually the most common options. It is currently a matter of debate, which surgical procedure to select for esophageal GISTs and their different clinical presentations (12-14). Other treatment strategies, in selected cases, have been proposed with good results, such as cryoablation or endoscopic resection (15,16).
Added to these diagnostic and therapeutic controversies, is the potential usefulness of using tyrosine kinase inhibitors (TKIs), such as imatinib, either neoadjuvant, achieving a reduction in the size of the lesion and favoring enucleation over esophagectomy or adjuvant, to reduce the risk of recurrence and improve survival (7,17-19).
Epidemiology and clinical presentation of esophageal GIST
GISTs are very infrequently located in the esophagus. In a review published in 2015 based on Surveillance, Epidemiology, and End Results Registry (SEER) data, esophageal GISTs accounted for only 23% of all esophageal sarcomas (behind carcinosarcoma and leiomyosarcoma), which represents 0.3% of all malignant tumors in this location and 1% of this same registry (8,18).
Among esophageal mesenchymal tumors, leiomyomas are the most frequent. On the other hand, GISTs account for approximately 25% of esophageal mesenchymal tumors (20). In an epidemiological analysis, only 0.7% of >9,700 GISTs recorded in studies published worldwide were esophageal (21).
As shown in some studies, esophageal GISTs predominantly affects males, with a mean age of 60 years (20,22,23). Along the same lines, a case series study (n=55) reported that esophageal GISTs were more frequent in men younger than 60 years, compared to gastric GISTs at the time of diagnosis (2).
Esophageal GISTs are most frequently located in the lower esophagus, followed by the middle esophagus and very rarely in the upper esophagus (2,24,25). Immunohistochemical studies have shown that ICCs are found predominantly in the distal esophagus, to a lesser extent in the middle esophagus, and very few in the upper esophagus (26). These histological findings were consistent with the distribution of esophageal GISTs (24).
A submucosal GISTs can have two growth patterns. On the one hand, it can extend towards the mucosa, or another possibility is to grow towards the serosa. In cases of extension to the mucosa, it can erode it and cause GI bleeding. Rupture or erosion of the serosa is rare and is usually secondary to trauma or intratumoral hemorrhage. Considering that the esophagus does not have a serosa, esophageal GISTs can rupture the pleura and bleed into the pleural cavity causing hemothorax. In the same way, they can also bleed into the peritoneal cavity and cause hemoperitoneum with acute thoracic or abdominal pain, depending on the case. Esophageal GISTs are not usually pedunculated unlike gastric GISTs, due to the narrow cylindrical shape of the organ (11).
The growth of an esophageal GIST can give early symptoms (70%), due to the narrowness of the organ. The most frequent symptom, given these circumstances, is dysphagia (36–51%), weight loss (20%), chest pain (8–15%) and less frequently bleeding (1–10%) (8). This fact may explain the relative younger age of these patients at the time of diagnosis. The remaining 30% of cases are asymptomatic and their diagnosis occurs incidentally on an esophagoscopy or barium esophagography (27).
Pathophysiology and molecular profiling
GISTs are positive for c-KIT (CD117) or CD34, and the ICCs, that are found in the myenteric plexus in the muscular layer of the GI tract, are known to be precursors of this entity (11).
To differentiate GISTs from other tumors, immunohistochemical assays including KIT (CD117), DOG1, CD34, smooth muscle actin (SMA), desmin, and S100 protein are performed (23,28,29). Histologically, esophageal GISTs vary from spindle cell (75%) to epithelioid (25%), and most of the reported tumors have been >5 cm or with >5 mitoses per 50 high-power fields (HPFs). Immunohistochemically, the reported tumors have been consistently positive for KIT and CD34 and occasionally positive for SMA (13%) and desmin (19%) (20).
Regarding the mutational study of esophageal GISTs, a study showed that most KIT mutations were found in exon 11. In this case series, the similarity in the mutational spectrum of esophageal GISTs compared with gastric GISTs was also observed. Of all cases with recurrent disease, KIT deletions in exon 11 involving codons 557 and/or 558 were demonstrated (23). Recent study have suggested that esophageal GISTs have an increased incidence of wild type (WT), compared to gastric GISTs (5).
Currently, risk stratification systems include tumor size, tumor location, tumor rupture, and mitotic activity (4,30-32). However, the ability to accurately determine the prognosis of patients with esophageal GISTs is unknown, because when these systems were established, few cases with esophageal GISTs were included in the risk assessment (7).
Despite the above, based on their size and mitotic index, esophageal GISTs are considered, in most cases, as tumors with a “high risk” (56–70%) of recurrence (2,23) due to their high risk of rupture or affected surgical margins. Similarly, according to the US National Cancer Database (NCDB), 53% of esophageal GISTs were “high risk” (5).
Diagnosis of esophageal GISTs
In all patients with suspected GISTs, the physical examination should include a detailed abdominal examination. GISTs may present as palpable abdominal masses either due to the primary tumor or intra-abdominal metastases (i.e., liver, omentum or peritoneum). Signs of acute abdomen or peritonitis may indicate the presence of GI bleeding, tumor rupture, bowel perforation, or GI obstruction. However, most patients with localized GISTs may have no specific physical exam findings, as some tumors present without symptoms, and extra-abdominal metastases are rare.
Upper GI oral contrast and upper GI endoscopy can help in the differential diagnosis between submucosal and mucosal lesions (33,34). On endoscopy, both GISTs and leiomyomas may appear as a submucosal mass with smooth margins, with a normal overlying mucosa, and bulging into the esophageal lumen. Central ulceration is occasionally seen.
Contrast-enhanced computed tomography (CT) of the thorax and abdomen is the imaging method of choice to characterize a thoraco-abdominal mass suspicious for GIST (Figure 1). Oral as well as intravenous contrast should be administered to define the bowel margins (35).
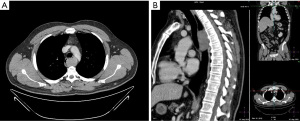
On CT, esophageal and esophagogastric junction GISTs appear as solid, homogeneous lesions that commonly enhance. Less frequently they show heterogeneity, due to tumor necrosis or intratumoral hemorrhage. As part of the differential diagnosis in a CT with suspected GIST of the esophagus or the stomach, leiomyoma, leiomyosarcoma, congenital cyst, carcinoma or neuroendocrine tumor should be included (36).
Although CT remains the preferred initial diagnostic imaging study for the evaluation of a suspected GIST, magnetic resonance imaging (MRI) of the thorax and abdomen has a similar diagnostic yield compared with CT and lacks radiation exposure. MRI may also be offered as an alternative to CT in patients who cannot receive intravenous CT contrast.
Recent studies have shown the utility of diffusion-weighted imaging (DWI) with the apparent diffusion coefficient (ADC) in differential diagnosis between uterine leiomyomas and leiomyosarcomas (37,38). Hihara et al. has proposed to use this new modality in the preoperative diagnosis of esophageal submucosal tumors (8).
Positron emission tomography-CT (PET-CT) has not replaced CT or MRI as the initial imaging modality of choice in patients suspected of having a GIST. PET-CT imaging is highly sensitive for detecting tumors with a high glucose metabolism, including GISTs; however, it is not sufficiently specific to make a preoperative diagnosis (39).
Endoscopic ultrasound (EUS) is useful to determine the size, shape, and intratumoral character of tumors and their relationships within the layers of the bowel wall (40). Unfortunately, distinguishing GISTs from leiomyomas by EUS findings is not generally possible (41).
On EUS, GISTs are typically hypoechoic, homogeneous lesions with well-defined margins, although they can rarely have irregular margins and ulcerations. Most GISTs originate from within the muscularis propria (fourth layer of the GI tract); small lesions may originate from the muscularis mucosa (second layer). Infrequently, the tumors are inhomogeneous, which has been attributed to liquefaction necrosis, connective tissue, and cystic and hyaline degeneration (40).
For the treatment of GISTs with TKIs, it is essential to have a histological diagnosis and its genetic study (2). The technique that allows an accurate differential diagnosis of a GIST as well as other mesenchymal tumors, is ultrasound-guided fine needle aspiration biopsy (FNAB). It has been shown to be a safe technique with minimal complications (42-44).
It is currently unclear whether biopsy or fine needle aspiration (FNA) is necessary in all cases. Especially in preoperative situations (11,20). Given the risks of hemorrhage, destruction of the pseudocapsula and subsequent tumor dissemination, in addition to causing scarring that makes enucleation of these submucosal lesions difficult, FNA is frequently avoided (25,27,41).
In contrast, the rationale for performing a preoperative biopsy, according to some published studies, is in tumors greater than 2 cm in diameter, lesions with an observed increase in size, and in patients with planned neoadjuvant treatment with TKI (2,13,42,43). According to the National Comprehensive Cancer Network (NCCN) Task Force Report, biopsy may not be necessary if the tumor is easily resectable and preoperative therapy is not required (3).
However, with the advances in immunohistochemistry and more frequent use of the EUS in diagnostic procedures, the sensitivity (78%) and specificity have increased, and tumors can be safely and feasibly biopsied. In a recent study, no intraoperative difficulties or tumor recurrence were reported in patients who were preoperatively biopsied (44).
Another indication for a FNAB with EUS, could be in cases where it is difficult to discriminate between GISTs and other esophageal stromal tumors (45).
Surgical therapy for esophageal GIST
Management guidelines for GISTs have been defined by consensus of the NCCN and the European Society of Medical Oncology (46-48). Although some basic surgical guidelines are enumerated by these panels, there have been no studies specifically addressing the issue of surgical resection for esophageal GISTs (42).
One of the important prognostic indicators in the treatment of GISTs is the complete exeresis of the disease, in addition to the location of the tumor, the size of the lesion, the indemnity of the pseudocapsula and the mitotic rate. R0 resection or complete resection of all macroscopic and microscopic disease, confirmed by pathology, has been associated with a better rate of local recurrence and overall survival in several published studies (49-51). Thus, during the planning and surgical procedure, complete exeresis should be a priority. In cases of GISTs with internal hemorrhage or necrosis, the consistency of these tumors can be quite friable, so all measures must be taken to avoid their intraoperative rupture, being basically interpreted as a positive surgical margin (11).
The surgical approach to GISTs can be performed by open surgery, by minimally invasive procedures such as thoracoscopy, laparoscopy, or even by a robotic approach (11). Regarding the need to perform a lymphadenectomy in esophageal GISTs, lymph node dissemination is very infrequent in this type of tumor. Therefore, routine lymph node dissection is not recommended (52,53). The surgical treatment of esophageal GISTs, due to their anatomical peculiarities, is primarily enucleation or esophagectomy, unlike gastric or intestinal GISTs, in which segmental or wedge resections can be performed (42).
Esophageal GISTs are more difficult to manage than are GISTs arising in serosa-lined intraabdominal organs because of the lack of tumor confinement by a serosal layer, and the relative contraindication to segmental resection given the blood supply of the esophagus (42,44).
The criteria to know which surgical procedure should be performed in esophageal GISTs is still under discussion (2,13,54,55). If postoperative morbidity and mortality are taken into account, tumor enucleation seems to be a better option, especially in fragile patients or with significant medical history (2,13,42,54). In general, in esophageal GISTs, enucleation is accepted in small tumors (2 to 5 cm in diameter), proposing esophagectomy in lesions greater than 9 cm in diameter or tumors with high-risk features (2,13,42,56). The oncological outcomes of these two procedures are reported to be similar with proper patient selection (7,13,42,44,56,57).
The optimal management of esophageal GISTs <2 cm in size is controversial. European Society for Medical Oncology (ESMO) guidelines recommend EUS and follow-up, reserving excision only for those esophageal nodules that increase in size (47). An algorithmic approach to management of GISTs ≤2 cm based upon size and EUS appearance has been proposed (58); however, this approach has not been prospectively validated. It has been adopted by the NCCN (48) for small gastric GISTs but not those at other sites.
Recent studies have published cases of enucleation and laparoscopic esophagectomy in esophageal GISTs with good results (7,59,60). The minimally invasive approach allows this type of intervention to be performed without undermining the oncological criteria established for GISTs and with undeniable advantages in terms of blood loss, hospital stay, and postoperative pain (57,61). The less invasive surgery might expand the indications for surgery, especially for smaller tumors and poor risk patients (8).
In cases of esophageal GISTs with mucosa ulceration and regardless of tumor size, esophagectomy is considered since enucleation is not technically possible. Other variables to keep in mind are the patient’s comorbidities and the morbidity of the procedure to be proposed, in addition to tumor diameter, location, and high-risk features (11).
Novel techniques such as cryoablation or endoscopic resection of esophageal GISTs have been published recently (15,16). These are generally the results of small, retrospective studies, including benign gastric lesions in addition to GISTs, and have limited data on long-term follow-up (62).
Continuous improvements in endoscopic luminal closure devices will probably increase the indication for endoscopic resection of gastric and esophageal GISTs in the coming years. Studies with sufficient evidence are needed to determine what type of patients will be the ideal candidates for this type of endoscopic resection (11).
Neoadjuvant therapy for esophageal GISTs
To date, there is little evidence based on clinical trials on neoadjuvant treatment of GISTs with imatinib (63). For esophageal GISTs, the available literature regarding neoadjuvant administration of imatinib is limited, and the usefulness of neoadjuvant therapy in these patients has been reported (7,19,64-66).
In patients with GISTs of the esophagus, duodenum or rectum, preoperative treatment with imatinib to reduce the size of the tumor and limit the extent of the resection may be a very interesting approach. Due to the anatomical complexity of these organs, the morbidity of the surgical procedures performed and the impact on the quality of life of patients due to loss of function, make this therapeutic strategy a frequently used option (8). If there is response to neoadjuvant treatment, laparoscopic surgery may be feasible. In some cases, laparoscopic and endoscopic cooperative surgery (LECS) should be useful in order to localize the tumor.
The duration of neoadjuvant or preoperative treatment with imatinib that has been published ranges from a few days to more than 12 months (7,67-69). To obtain a maximum response before surgery, the optimal duration of imatinib before surgery is considered to be 6 to 12 months (48). However, careful follow-up during preoperative treatment with imatinib is important, as an increased risk of rupture or bleeding due to tumor necrosis and cystic changes has been described (1,63).
In patients with GISTs with high mitotic rates or large tumors, neoadjuvant treatment with imatinib can help achieve complete resection (R0) and also reduce intraoperative complications, including pseudocapsular rupture (23).
Adjuvant therapy for esophageal GISTs
Following GIST excision, adjuvant TKI treatment has been shown to decrease recurrences and increase survival in many clinical studies (70,71). However, due to its low incidence, esophageal GISTs were not included in these studies and more studies are needed to compare the efficacy of adjuvant therapy with imatinib in these cases (1).
Adjuvant imatinib recurrence-free survival benefit depends on the location of the mutations, with those involving KIT exon 11 codons 557 and/or 558 seeming to benefit most (72-74).
Low and intermediate risk GISTs do not require adjuvant treatment, whereas high risk GISTs with mutations sensitive to imatinib are usually treated with three years of imatinib based on published data (72,75-77). Recently data from new molecules as avapritinib and ripretinib show encouraging data for the treatment of patients with mutations resistant to imatinib (47) in advanced or metastatic tumors. GIST with PDGFRA-D842V mutation, neoadjuvant treatment with avapritinib may be an option.
Clinical outcome of esophageal GISTs
The overall survival observed in different series is 45–85% at 5 years, with a recurrence rate of 22–39% (2,5,23-25). The main risk factors are tumor size (>5 cm) and mitotic index (>5 mitoses per 50 HPFs). Compared with gastric GIST, and equal in size, mitosis and adjuvant treatment, the prognosis of esophageal GIST is noticeably worse (2,24). It is often argued that the absence of esophageal serosa favors metastatic spread in esophageal GIST, which would explain that the percentage of cases with metastases is higher (22–39%), especially when compared with a 26% in small intestine GIST or 9% in the rectum (61).
Compared with esophageal cancer, recurrence in esophageal GIST can manifest beyond 5 years after surgical resection (25), which requires a long follow-up in these patients. Liver metastases, followed by lung, thoracic cavity, pleura, peritoneum, and subcutaneous (24) are common sites of systemic recurrence.
The need for esophagectomy is usually identified as a risk factor for recurrence, but this variable is related to tumor size as seen above, since the larger the size, the more likely it is to perform an esophagectomy to complete a R0 resection. In this sense, one study reports a recurrence rate of 33% in esophagectomies (large GISTs) compared to 0% in enucleations (smaller GISTs).
In case of tumor rupture or R1 resection, and regardless of the surgical technique (esophagectomy or enucleation), the recurrence rate is between 35% and 50%, while it is 0% in the absence of these factors (20,22,23,44).
The reported recurrence rates in others publications are quite approximate between enucleation (42.4% and 52.8%) and esophagectomy (57.6% and 47.7%), so there must be additional factors that explain these data (2,12). In line with the above, a study identified, in addition to a size ≥10 cm and a mitotic index >5 mitoses per 50 HPFs, the presence of deletion-type mutations in exon 11 at codons 557–558 and R1 margins as risk factors additional recurrence (23).
Recommendations in esophageal GIST
- Preoperative biopsy is necessary to avoid diagnostic errors.
- Neoadjuvant imatinib (TKI) treatment is recommended in big tumors (>10 cm) and tumors located in the esophagogastric junction.
- In high-risk tumors, three years adjuvant therapy is recommended.
- If it is feasible, minimally invasive approach is preferred.
- Esophagectomy should be avoided if enucleation is allowing an oncological resection.
Rectal GIST
The histological characteristics, the size, location, and extension of rectal GISTs determine the oncological prognosis and the therapeutic attitude. That is why a meticulous study must be carried out using imaging tests (CT scan, pelvic MRI, transrectal ultrasound) to assess these variables and establish an adjusted treatment plan within a multidisciplinary oncology committee. Pelvic MRI is useful for defining anatomical limits of the tumor and design the surgical strategy.
A wide range of nonspecific symptoms can be present: abdominal pain, rectal bleeding, a feeling of tenesmus or occupation. Occasionally they can cause abdominal distention in more advanced stages.
Although the diagnosis is sometimes incidental due to imaging tests for different reasons, the initial diagnosis of suspicion is often due to a colonoscopy, in which a submucosal mass is appreciated. The endoscopic diagnosis is made based in a subepithelial tumor with normal overlying mucosa (63.1%), followed by a subepithelial tumor with erosion, ulceration, or bleeding (31.6%) (78).
The suspected diagnosis must be confirmed by obtaining a biological sample for histological study. If sufficient material is not obtained by endoscopy, in the event of a high suspicion of a GI tumor, a guided puncture by transrectal ultrasound is indicated.
Surgical management of rectal GIST
Surgery is the curative treatment in rectal GIST. Local resection, if complete resection can be achieved, should be the first option. When the neoplasm affects the sphincters in such a way that they cannot be preserved, radical surgery through abdominoperineal resection should be performed.
The anatomical characteristics of the pelvis, largely determine the treatment. As it is a rigid and narrow structure, the growth of the tumor can compromise the resectability of the lesion earlier than in other locations. Obtaining a specimen with free surgical margins can be limited if the tumor reaches the sidewalls of the pelvis. Likewise, the growth of lesions towards anterior compartments can reach neighboring organs, both the vagina or uterus in women, and the prostate in men.
On the other hand, the involvement of the sphincters of the anus can often lead to radical resection of the sphincter complex, a circumstance that compromises its preservation, with the eventual need for a terminal stoma.
Lymph node involvement in GIST is extremely rare. Therefore, surgery with curative intention does not include the need for lymphadenectomy, and mesorectal excision, is not necessary in case of GIST. However, the limited space in this anatomical region sometimes makes it impossible to approach these tumors locally, especially in voluminous lesions, requiring anterior resection of the rectum or abdominoperineal resection.
Different studies have shown that local resection, if it achieves clear surgical margins, has a similar prognosis as extended resections, with a lower rate of complications, shorter operative time (79) and better quality of life (80).
There are many approaches described for local resection of rectal GIST, the most used being the transanal approach (Figure 2). In this approach, different colorectal techniques have been developed, from classical or conventional transanal surgery, to Natural Orifice Transluminal Endoscopic Surgery (NOTES) techniques, whose endoscopic approach and different platforms or working channels can be used [transanal endoscopic microsurgery (TEM), transanal endoscopic operation (TEO) or transanal minimal invasive surgery (TAMIS)]. Although TAMIS is currently the most widely used technique for local resection, there are other approaches described in the literature.
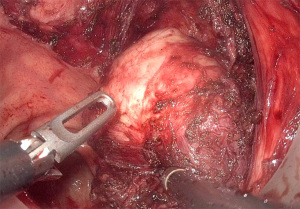
The TEM techniques, in their different variants, compared to the conventional technique not assisted by endoscopy, improve the visualization of the surgical field because of the magnification and stabilization of the image. The use of TEM in the treatment of rectal tumors, can avoid radical surgery (81).
The para-sacral or trans-sacral approaches are classic techniques in the treatment of other rectal pathologies, and they have also been used for the resection of rectal GISTs (82,83). They may be of special interest when the tumor is voluminous, posterior, and reaches the superior part of the puborectalis muscle, especially if transanal surgery does not guarantee adequate resection.
The vaginal approach is also described, used for lower rectum GIST located behind the posterior wall of the vagina (84).
In cases of advanced local growth and involvement of organs close to the rectum, more extended resections may be necessary, and the abdominal approach is frequently used. In these more advanced cases, anterior rectal resection with multivisceral resection may be necessary and can be performed with or without preservation of the anal sphincters.
Neoadjuvant treatment for rectal GIST
Although treatment with imatinib demonstrated its benefit as an adjuvant treatment 20 years ago, its use as neoadjuvant therapy in rectal GIST has been evaluated more recently.
The first objective of “multimodal” management through neoadjuvant treatment is to reduce the size of the lesion, achieving a less mutilating surgical technique, as well as reducing the chances of tumor rupture. Secondarily, a better rate of sphincter-sparing surgery can be achieved. Both the guidelines of the ESMO (47) and the NCCN guidelines (48) recommend its use in those cases in which there is a risk of not obtaining a free resection surgical margin or there is a risk of tumor rupture. Thus, it is widely accepted that the choice to perform neoadjuvant therapy should be carried out in the context of a multidisciplinary committee.
The duration of neoadjuvant treatment is not clearly established and there are studies with different durations of treatment, between 6 and 12 months. This duration must be decided within the multidisciplinary committee. The efficacy is variable, the complete response being infrequent 4.2%, with 66% of patients with a partial response, with stabilization of the disease in 30% and progression in slightly less than 1% (85). Sphincter sparing is more frequent after neoadjuvant treatment 33–94% vs. 14%. Rectal sparing also is more frequent in the setting of neoadjuvant treatment with imatinib (86). The application of neoadjuvant significantly decreased tumor size in large rectal GIST and may allow TEM to preserve sphincter (81). Endoscopic surgeries with neoadjuvant treatment are safe for patients with rectal GIST and increase the rate of anus and anal function preservation.
The assessment of the response to treatment can be carried out mainly through radiological controls. In this sense, tumor size shrinking in the CT scan has been the most used variable. It is also possible to verify that the decrease in the density of the tumor tissue in the radiological tests can be a sign of response, even an earlier data to the reduction of the tumor volume. On the other hand, PET-CT has been highly sensitive in early assessments of response to treatment (47).
Different studies have investigated the efficacy of neoadjuvant therapy with Imatinib in relation to the different locations of the KIT mutation, showing different response rates (87-90).
Clinical outcomes of rectal GIST
There is currently no established general recommendation for the follow-up of rectal GIST. Most recurrences are diagnosed in the first three years, although low-grade tumors can progress more slowly and be diagnosed later. Overall, it is accepted that a follow-up adjusted to the risk of GIST (according to the mitosis index and tumor size) can be carried out. Based on these variables, CT scans can be performed every 3 or 4 months for the first 3 years and then every 6 months until completing 5 years of follow-up (47).
In a recent study carried out in Europe that collected patients with rectal GIST in five different hospitals between 2009 and 2018, 155 patients were analyzed after surgery, in which 65% received neoadjuvant treatment. The local recurrence rate was 15% and the global recurrence rate 28%, with no differences regarding the surgical technique (local or radical) (91). However, there are several studies that demonstrate an improvement in disease-free and recurrence-free survival after the introduction of Imatinib as a neoadjuvant treatment (86,92).
Recommendations in rectal GIST
- Local resection is recommended in low-risk tumors, <2 cm in which sphincters are not affected.
- Surgery with curative intention does not include the need for lymphadenectomy, and mesorectal excision, is not necessary in case of GIST.
- Neoadjuvant treatment is recommended in those cases in which there is a risk of not obtaining a free resection surgical margin or there is a risk of tumor rupture.
- CT scans can be performed every 3 or 4 months for the first 3 years and then every 6 months until completing 5 years of follow-up.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Claudia Valverde, Nadia Hindi) for the series “Management of Gastrointestinal Stromal Tumors” published in Gastrointestinal Stromal Tumor. The article has undergone external peer review.
Peer Review File: Available at https://gist.amegroups.com/article/view/10.21037/gist-22-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gist.amegroups.com/article/view/10.21037/gist-22-2/coif). The series “Management of Gastrointestinal Stromal Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang FB, Shi HC, Shu YS, et al. Diagnosis and surgical treatment of esophageal gastrointestinal stromal tumors. World J Gastroenterol 2015;21:5630-4. [Crossref] [PubMed]
- Lott S, Schmieder M, Mayer B, et al. Gastrointestinal stromal tumors of the esophagus: evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am J Cancer Res 2015;5:333-43. [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8:S1-S44. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Briggler AM, Graham RP, Westin GF, et al. Clinicopathologic features and outcomes of gastrointestinal stromal tumors arising from the esophagus and gastroesophageal junction. J Gastrointest Oncol 2018;9:718-27. [Crossref] [PubMed]
- Ma GL, Murphy JD, Martinez ME, et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev 2015;24:298-302. [Crossref] [PubMed]
- Neofytou K, Costa Neves M, Giakoustidis A, et al. Effective Downsizing of a Large Oesophageal Gastrointestinal Stromal Tumour with Neoadjuvant Imatinib Enabling an Uncomplicated and without Tumour Rupture Laparoscopic-Assisted Ivor-Lewis Oesophagectomy. Case Rep Oncol Med 2015;2015:165736. [Crossref] [PubMed]
- Hihara J, Mukaida H, Hirabayashi N. Gastrointestinal stromal tumor of the esophagus: current issues of diagnosis, surgery and drug therapy. Transl Gastroenterol Hepatol 2018;3:6. [Crossref] [PubMed]
- Yan W, Zhang A, Powell MJ. Genetic alteration and mutation profiling of circulating cell-free tumor DNA (cfDNA) for diagnosis and targeted therapy of gastrointestinal stromal tumors. Chin J Cancer 2016;35:68. [Crossref] [PubMed]
- Bischof DA, Kim Y, Dodson R, et al. Conditional disease-free survival after surgical resection of gastrointestinal stromal tumors: a multi-institutional analysis of 502 patients. JAMA Surg 2015;150:299-306. [Crossref] [PubMed]
- Theiss L, Contreras CM. Gastrointestinal Stromal Tumors of the Stomach and Esophagus. Surg Clin North Am 2019;99:543-53. [Crossref] [PubMed]
- Pence K, Correa AM, Chan E, et al. Management of esophageal gastrointestinal stromal tumor: review of one hundred seven patients. Dis Esophagus 2017;30:1-5. [Crossref] [PubMed]
- Lee HJ, Park SI, Kim DK, et al. Surgical resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2009;87:1569-71. [Crossref] [PubMed]
- Cohen C, Pop D, Icard P, et al. Is There a Place for Thoracoscopic Enucleation of Esophageal Gastrointestinal Stromal Tumors? Thorac Cardiovasc Surg 2019;67:585-8. [Crossref] [PubMed]
- Mai D, Hashimoto R, Yu A, et al. Successful Curative Cryoablation of an Esophageal Gastrointestinal Stromal Tumor. ACG Case Rep J 2019;6:e00076. [Crossref] [PubMed]
- Jiao R, Zhao S, Jiang W, et al. Endoscopic Submucosal Dissection of Gastrointestinal Stromal Tumours: A Retrospective Cohort Study. Cancer Manag Res 2020;12:4055-61. [Crossref] [PubMed]
- Poveda A, García Del Muro X, López-Guerrero JA, et al. GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev 2017;55:107-19. [Crossref] [PubMed]
- Wu GX, Ituarte PH, Paz IB, et al. A Population-Based Examination of the Surgical Outcomes for Patients with Esophageal Sarcoma. Ann Surg Oncol 2015;22:S1310-7. [Crossref] [PubMed]
- Yanagawa S, Tanabe K, Suzuki T, et al. A large esophageal gastrointestinal stromal tumor that was successfully resected after neoadjuvant imatinib treatment: case report. World J Surg Oncol 2014;12:47. [Crossref] [PubMed]
- Miettinen M, Sarlomo-Rikala M, Sobin LH, et al. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol 2000;24:211-22. [Crossref] [PubMed]
- Søreide K, Sandvik OM, Søreide JA, et al. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016;40:39-46. [Crossref] [PubMed]
- Duffaud F, Meeus P, Bertucci F, et al. Patterns of care and clinical outcomes in primary oesophageal gastrointestinal stromal tumours (GIST): A retrospective study of the French Sarcoma Group (FSG). Eur J Surg Oncol 2017;43:1110-6. [Crossref] [PubMed]
- Kang G, Kang Y, Kim KH, et al. Gastrointestinal stromal tumours of the oesophagus: a clinicopathological and molecular analysis of 27 cases. Histopathology 2017;71:805-12. [Crossref] [PubMed]
- Feng F, Tian Y, Liu Z, et al. Clinicopathologic Features and Clinical Outcomes of Esophageal Gastrointestinal Stromal Tumor: Evaluation of a Pooled Case Series. Medicine (Baltimore) 2016;95:e2446. [Crossref] [PubMed]
- Nakano A, Akutsu Y, Shuto K, et al. Giant esophageal gastrointestinal stromal tumor: report of a case. Surg Today 2015;45:247-52. [Crossref] [PubMed]
- Radenkovic G, Ilic I, Zivanovic D, et al. C-kit-immunopositive interstitial cells of Cajal in human embryonal and fetal oesophagus. Cell Tissue Res 2010;340:427-36. [Crossref] [PubMed]
- Nemeth K, Williams C, Rashid M, et al. Oesophageal GIST-A rare breed case report and review of the literature. Int J Surg Case Rep 2015;10:256-9. [Crossref] [PubMed]
- Yamaguchi U, Hasegawa T, Masuda T, et al. Differential diagnosis of gastrointestinal stromal tumor and other spindle cell tumors in the gastrointestinal tract based on immunohistochemical analysis. Virchows Arch 2004;445:142-50. [Crossref] [PubMed]
- Kang GH, Srivastava A, Kim YE, et al. DOG1 and PKC-θ are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Mod Pathol 2011;24:866-75. [Crossref] [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Levy AD, Remotti HE, Thompson WM, et al. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics 2003;23:283-304, 456; quiz 532. [Crossref] [PubMed]
- Sharp RM, Ansel HJ, Keel SB. Best cases from the AFIP: gastrointestinal stromal tumor. Armed Forces Institute of Pathology. Radiographics 2001;21:1557-60. [Crossref] [PubMed]
- Tateishi U, Hasegawa T, Satake M, et al. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr 2003;27:792-8. [Crossref] [PubMed]
- Lewis RB, Mehrotra AK, Rodriguez P, et al. From the radiologic pathology archives: esophageal neoplasms: radiologic-pathologic correlation. Radiographics 2013;33:1083-108. [Crossref] [PubMed]
- Sato K, Yuasa N, Fujita M, et al. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol 2014;210:368.e1-8. [Crossref] [PubMed]
- Tasaki A, Asatani MO, Umezu H, et al. Differential diagnosis of uterine smooth muscle tumors using diffusion-weighted imaging: correlations with the apparent diffusion coefficient and cell density. Abdom Imaging 2015;40:1742-52. [Crossref] [PubMed]
- Kim SJ, Lee SW. Performance of F-18 FDG PET/CT for predicting malignant potential of gastrointestinal stromal tumors: A systematic review and meta-analysis. J Gastroenterol Hepatol 2018;33:576-82. [Crossref] [PubMed]
- Săftoiu A. Endoscopic ultrasound-guided fine needle aspiration biopsy for the molecular diagnosis of gastrointestinal stromal tumors: shifting treatment options. J Gastrointestin Liver Dis 2008;17:131-3. [PubMed]
- Dendy M, Johnson K, Boffa DJ. Spectrum of FDG uptake in large (>10 cm) esophageal leiomyomas. J Thorac Dis 2015;7:E648-51. [PubMed]
- Blum MG, Bilimoria KY, Wayne JD, et al. Surgical considerations for the management and resection of esophageal gastrointestinal stromal tumors. Ann Thorac Surg 2007;84:1717-23. [Crossref] [PubMed]
- Stelow EB, Stanley MW, Mallery S, et al. Endoscopic ultrasound-guided fine-needle aspiration findings of gastrointestinal leiomyomas and gastrointestinal stromal tumors. Am J Clin Pathol 2003;119:703-8. [Crossref] [PubMed]
- Robb WB, Bruyere E, Amielh D, et al. Esophageal gastrointestinal stromal tumor: is tumoral enucleation a viable therapeutic option? Ann Surg 2015;261:117-24. [Crossref] [PubMed]
- Schizas D, Bagias G, Kanavidis P, et al. Prognostic factors affecting mortality in patients with esophageal GISTs. J BUON 2020;25:497-507. [PubMed]
- Ahmed M. Recent advances in the management of gastrointestinal stromal tumor. World J Clin Cases 2020;8:3142-55. [Crossref] [PubMed]
- Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022;33:20-33. [Crossref] [PubMed]
- von Mehren M, Kane JM, Bui MM, et al. Gastrointestinal stromal tumors (GISTs). NCCN Guidelines; 2020:1-44.
- Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992;215:68-77. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Aparicio T, Boige V, Sabourin JC, et al. Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol 2004;30:1098-103. [Crossref] [PubMed]
- Matsumoto S, Takayama T, Wakatsuki K, et al. An esophageal gastrointestinal stromal tumor with regional lymph node metastasis. Esophagus 2010;7:115-8. [Crossref]
- Masuda T, Toh Y, Kabashima A, et al. Overt lymph node metastases from a gastrointestinal stromal tumor of the esophagus. J Thorac Cardiovasc Surg 2007;134:810-1. [Crossref] [PubMed]
- Coccolini F, Catena F, Ansaloni L, et al. Esophagogastric junction gastrointestinal stromal tumor: resection vs enucleation. World J Gastroenterol 2010;16:4374-6. [Crossref] [PubMed]
- Peparini N, Carbotta G, Chirletti P. Enucleation for gastrointestinal stromal tumors at the esophagogastric junction: is this an adequate solution? World J Gastroenterol 2011;17:2159-60. [Crossref] [PubMed]
- Jiang P, Jiao Z, Han B, et al. Clinical characteristics and surgical treatment of oesophageal gastrointestinal stromal tumours. Eur J Cardiothorac Surg 2010;38:223-7. [Crossref] [PubMed]
- von Rahden BH, Stein HJ, Feussner H, et al. Enucleation of submucosal tumors of the esophagus: minimally invasive versus open approach. Surg Endosc 2004;18:924-30. [Crossref] [PubMed]
- Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol 2009;6:363-71. [Crossref] [PubMed]
- Isaka T, Kanzaki M, Onuki T. Long-term survival after thoracoscopic enucleation of a gastrointestinal stromal tumor arising from the esophagus. J Surg Case Rep 2015;2015:rju155. [Crossref] [PubMed]
- Koyanagi K, Nakagawa M, Ozawa S, et al. Thoracoscopic enucleation for small-sized gastrointestinal stromal tumor of the esophagus: report of two cases. Esophagus 2010;7:219-24. [Crossref]
- Azab B, Macedo FI, Cass SH, et al. A large national comparative study of clinicopathological features and long-term survivals between esophageal gastrointestinal stromal tumor and leiomyosarcoma. Am J Surg 2019;218:323-8. [Crossref] [PubMed]
- He G, Wang J, Chen B, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc 2016;30:4206-13. [Crossref] [PubMed]
- Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012;19:1074-80. [Crossref] [PubMed]
- Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 2009;35:739-45. [Crossref] [PubMed]
- Shinagare AB, Zukotynski KA, Krajewski KM, et al. Esophageal gastrointestinal stromal tumor: report of 7 patients. Cancer Imaging 2012;12:100-8. [Crossref] [PubMed]
- Sato H, Kanda T, Hirota S, et al. Surgical resection of gastrointestinal stromal tumor of esophagus following preoperative imatinib treatment: A case report. Esophagus 2010;7:65-9. [Crossref]
- Bednarski BK, Araujo DM, Yi M, et al. Analysis of prognostic factors impacting oncologic outcomes after neoadjuvant tyrosine kinase inhibitor therapy for gastrointestinal stromal tumors. Ann Surg Oncol 2014;21:2499-505. [Crossref] [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [Crossref] [PubMed]
- Tielen R, Verhoef C, van Coevorden F, et al. Surgery after treatment with imatinib and/or sunitinib in patients with metastasized gastrointestinal stromal tumors: is it worthwhile? World J Surg Oncol 2012;10:111. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial. JAMA Oncol 2017;3:602-9. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD. Defining the Impact of Adjuvant Therapy in Molecularly Defined Subsets of Gastrointestinal Stromal Tumor: From Lumping to Splitting. JAMA Oncol 2017;3:597-9. [Crossref] [PubMed]
- Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016;34:244-50. [Crossref] [PubMed]
- Le Cesne A, Blay JY, Reichardt P, et al. Optimizing tyrosine kinase inhibitor therapy in gastrointestinal stromal tumors: exploring the benefits of continuous kinase suppression. Oncologist 2013;18:1192-9. [Crossref] [PubMed]
- Marrari A, Wagner AJ, Hornick JL. Predictors of response to targeted therapies for gastrointestinal stromal tumors. Arch Pathol Lab Med 2012;136:483-9. [Crossref] [PubMed]
- Kim JB, Ye BD, Lee JL, et al. Rectal gastrointestinal stromal tumor: clinical features, endoscopic findings and prognosis. Hepatogastroenterology 2014;61:70-5. [PubMed]
- Guo W, Yang Z, Wei Y, et al. Radical excision versus local resection for primary rectal gastrointestinal stromal tumors. Cohort Study. Int J Surg 2020;77:190-7. [Crossref] [PubMed]
- Changchien CR, Wu MC, Tasi WS, et al. Evaluation of prognosis for malignant rectal gastrointestinal stromal tumor by clinical parameters and immunohistochemical staining. Dis Colon Rectum 2004;47:1922-9. [Crossref] [PubMed]
- Bai X, Zhou W, Li Y, et al. Transanal endoscopic microsurgery with alternative neoadjuvant imatinib for localized rectal gastrointestinal stromal tumor: a single center experience with long-term surveillance. Surg Endosc 2021;35:3607-17. [Crossref] [PubMed]
- Matsumi Y, Hamada M, Sakaguchi T, et al. Para-sacral approach followed by laparoscopic low anterior resection of a gastrointestinal stromal tumour at the anterior wall of the lower rectum. Colorectal Dis 2021;23:1579-83. [Crossref] [PubMed]
- Tazawa H, Hirata Y, Kuga Y, et al. Sphincter-saving resection by cluneal arched skin incision for a gastrointestinal stromal tumor (GIST) of the lower rectum: a case report. Surg Case Rep 2017;3:8. [Crossref] [PubMed]
- Shizhuo W. Transvaginal excision of rectal stromal tumors: case reports and a literature review. World J Surg Oncol 2019;17:164. [Crossref] [PubMed]
- Kaneko M, Emoto S, Murono K, et al. Neoadjuvant imatinib therapy in rectal gastrointestinal stromal tumors. Surg Today 2019;49:460-6. [Crossref] [PubMed]
- Cavnar MJ, Wang L, Balachandran VP, et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann Surg Oncol 2017;24:3972-80. [Crossref] [PubMed]
- Huynh TK, Meeus P, Cassier P, et al. Primary localized rectal/pararectal gastrointestinal stromal tumors: results of surgical and multimodal therapy from the French Sarcoma group. BMC Cancer 2014;14:156. [Crossref] [PubMed]
- Liu H, Yan Z, Liao G, et al. Treatment strategy of rectal gastrointestinal stromal tumor (GIST). J Surg Oncol 2014;109:708-13. [Crossref] [PubMed]
- Pai VD, Demenezes JL, Patil PS, et al. Multimodality therapy of rectal gastrointestinal stromal tumors in the era of imatinib-an Indian series. J Gastrointest Oncol 2016;7:262-8. [PubMed]
- Wilkinson MJ, Fitzgerald JE, Strauss DC, et al. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br J Surg 2015;102:965-71. [Crossref] [PubMed]
- IJzerman NS, Mohammadi M, Tzanis D, et al. Quality of treatment and surgical approach for rectal gastrointestinal stromal tumour (GIST) in a large European cohort. Eur J Surg Oncol 2020;46:1124-30. [Crossref] [PubMed]
- Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 2013;20:586-92. [Crossref] [PubMed]
Cite this article as: Asencio JM, Jiménez LM, Steiner MA. Rectal and esophageal gastrointestinal stromal tumors—how to deal with it?—technical approach and role of neoadjuvant treatment. Gastrointest Stromal Tumor 2023;6:2.


