
Which is the Achilles heel of gastrointestinal stromal tumour and how to use it for the future?—a review of novel therapeutic paradigms
Introduction: gastrointestinal stromal tumour (GIST) biological background
GIST, with an annual incidence of 11–19.6 cases per million, is the most common malignant mesenchymal neoplasm (1). It arises from the interstitial cells of Cajal (ICCs), which extend throughout the gastrointestinal (GI) tract and coordinate peristaltic movements (2). Normal functioning of ICCs depends on activation of the receptor tyrosine kinase (RTK) KIT. Gain-of-function mutations in KIT are the oncogenic driver for about 80% of all GISTs. KIT is a member of the type-III RTK family receptor, which includes platelet-derived growth factor receptor alpha (PDGFRA) and whose mutations drive about 10% of all GISTs (3,4).
Normal KIT activation occurs upon binding of its ligand, stem cell factor (SCF), that causes receptor homodimerization and consequent kinase activation (2). In GIST, KIT mutations lead to ligand-independent KIT activation. Two thirds of all GISTs have primary mutations in the juxtamembrane domain encoded by exon 11. These mutations hinder the ability of this domain to auto-inhibit KIT, which it would normally do by preventing the activation loop from assuming the active conformation necessary for kinase activation (5). Occasionally, primary mutations occur in exon 9 that affect the extracellular loop of KIT (6). In rare occasions, the ATP-binding pocket (BP) or the activation loop are affected by KIT mutations in exons 13 and 17, respectively (7). GISTs driven by PDGFRA most commonly have primary mutations in exon 18, affecting the activation loop domain, but may also occur in exons 12 and 14 that encode, respectively, for the juxtamembrane and ATP-BP domain (4,8).
Upon KIT and PDGFRA mutations, the RAS/MAPK and PI3K/AKT/mTOR pathways are constitutively activated, becoming vital oncogenic pathways throughout the course of the disease (9-11). Importantly, the transcription factor (TF) ETS translocation variant 1 (ETV1) is highly expressed in GIST and has been shown to be essential for GIST tumorigenesis and progression. It is stabilised by the MAPK pathway upon KIT activity (12). Genomic events that dysregulate the RAS/MAPK pathway can supplant the pathway initiated by KIT, which may contribute to treatment resistance upon KIT-inhibition, highlighting the importance of this pathway (13). Additionally, the PI3K pathway is crucial for GIST tumorigenesis and survival, and it is also a key player in the clinical outcome of GIST treatments (9,11,14). For this reason, and to great success, GIST treatments have focused on exploiting this oncogenic addiction.
This rationale was successfully utilized in 2001 upon approval of the type II tyrosine kinase inhibitor (TKI) imatinib. A remarkable 90% of GIST patients experience a clinical benefit after treatment, showing a median progression-free survival (mPFS) of 20–24 months (15). While outstanding for a disease with no previous treatment, most patients eventually develop resistance due to the polyclonal expansion of tumour subpopulations harbouring secondary KIT mutations. These additional mutations are substitutions that affect two domains: the ATP-BP and the activation loop. ATP-BP mutations arise in exons 13 and 14, while those affecting the activation loop in exons 17 and 18. They are the instigators of imatinib drug resistance by directly interfering with drug binding to the receptor (16-18). GISTs driven by PDGFRA are normally unresponsive to imatinib, as their most common mutation, D842V, disfavours imatinib binding—as well as binding of most approved therapies (19). Additional lines of treatment for GIST have focused on the development of multikinase inhibitors with broader activity against KIT oncoproteins to circumvent this resistance. Multikinase inhibitors sunitinib and regorafenib are the standard second-and third-line treatment options for metastatic GIST patients (19,20). Unfortunately, neither therapy offers a prolonged response, nor can they effectively treat PDGFRA driven GISTs. In 2020, however, two additional therapies were approved by the FDA, offering a more promising outlook. Ripretinib is a type II TKI specifically designed to target a wide range of primary and secondary mutations in GIST, making it the first treatment that can effectively treat GISTs irrespective of their mutational status, though to varying degrees. On the other hand, avapritinib is a type I TKI that targets GISTs with KIT or PDGFRA mutations in the activation loop. Remarkably, avapritinib is able to successfully treat PDGFRA mutations, such as the D842V substitution, being the first GIST treatment to be able to do so.
Targeted therapies with a single agent often lead to emergence of resistant clones, which severely limit their efficacy. While targeting GIST’s oncogenic addiction has proven to be a successful and viable therapeutic strategy that must continue being exploited, there is a need for diverse treatment approaches. Importantly, due to the heterogeneity in secondary resistance mutations across patients, but also within individual patients and lesions, there is a need to develop further treatments that are independent of KIT mutational status (18-22).
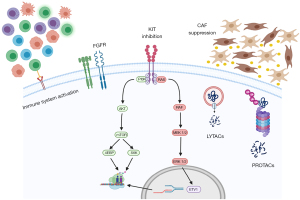
Here, we will discuss novel strategies that have been developed—or that are being developed—to treat different cancers and explain why these approaches need to be explored in GIST. This is based on our own laboratory experience, ongoing research projects and the most updated literature review. Specifically, we will talk about the importance of the ubiquitin-proteasome system (UPS) in GIST and why targeting this pathway is of therapeutic relevance in GIST. Related, we will discuss recent approaches in drug development that hijack the normal cellular protein homeostasis mechanisms in what is called targeted protein degradation and why these therapies could represent breakthroughs in GIST. Additionally, the role of the (immune) microenvironment in GIST will be discussed to bring forth its relevance in future GIST therapeutics. Lastly, we will mention recent approaches aimed at targeting GIST’s therapeutic adaptation and a novel drug that targets GIST independently of its mutational status. Therefore, the Achilles of heel of GIST is not a single agent. Instead, the arrows needed to defeat GIST are a combination of different approaches, targeting diverse parts of GIST biology. All these therapeutic strategies are represented in Figure 1. We present the following article in accordance with the Narrative Review reporting checklist (available at https://gist.amegroups.com/article/view/10.21037/gist-21-11/rc).
Methods
PubMed search, all years considered but articles only in English language (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 30/04/2021 |
| Databases and other sources searched | PubMed |
| Search terms used | GIST, UPS, ubiquitin, cancer, microenvironment, immune system |
| Timeframe | All years considered |
| Inclusion criteria | English only; research and review articles |
| Selection process | Search and consensus by Iván Olivares-Rivas |
GIST, gastrointestinal stromal tumour; UPS, ubiquitin-proteasome system.
UPS
Around 80–90% of intracellular protein degradation is undertaken by the UPS, while the remainder proteins are degraded by the lysosome. The components of the UPS are: ubiquitin (Ub), Ub-activating enzymes (E1s), Ub-conjugating enzymes (E2s), Ub ligases (E3s), the proteosome and deubiquitinases (DUBs) (23). Protein substrates are modified with ubiquitin, serving as a tag that is then recognised by 26s proteosomes responsible for proteolysis of the substrate. Ubiquitin is conjugated to the substrate via a multistep cascade involving E1, E2 and E3 enzymes. Concisely, E1 enzymes activate Ub through an ATP-dependent thioester bond between the C-terminal of Ub and a Cys residue in the catalytic site of the E1 enzyme. Ub is then transferred to an E2 enzyme, forming also a thioester bond between them, after which, through cooperation between an E2 and an E3 enzyme, the activated Ub is transferred to a lysine (Lys) target substrate (23).
Protein degradation is undertaken by the 26S proteasome, which is a 2.5 MDa multiprotein complex. It contains a 20S tube-like proteolytic core particle and two 19S regulatory particles at either end (24). Ubiquitin contains seven Lys residues, all of which can covalently attach to other ubiquitins and form linear or branched Ub chains. The type of Lys residue involved in the formation of these poly-Ub chains dictates the consequence of the ubiquitination. Lys48-linked-poly-Ub chains mark proteins for proteasomal degradation, while Lys63-linked-poly-Ub chains are associated with non-proteasomal signalling like endocytic trafficking, DNA replication and signal transduction (25).
In the human proteome there are two E1 isoforms and 40 E2 enzymes, while the repertoire of E3 ligases is vast, with an estimated 600 members. E3 enzymes are divided in three groups according to their Ub transfer mechanism from an E2 enzyme to the substrate: (I) the ‘really interesting new gene’ (RING) class, (II) the ‘homologous to E6-AP carboxyl terminus’ (HECT) class, and (III) the ‘RING-between-RING’ (RBR) class (26). RING E3 ligases encompass the largest family. They mediate the transfer of Ub from the E2 enzyme to the substrate without forming bonds with Ub (27). On the other hand, HECT and RBR ligases, via distinct mechanisms, form intermediate thioester bonds with the Ub attached to the E2 to facilitate Ub transfer to the substrate (25,27). RING E3 ligases can be subdivided into single subunit or multi-subunit E3s. Cullin-RING-Ub ligases (CRLs) exemplify multi-subunit E3s, which allow one core scaffold to regulate the ubiquitylation of diverse substrates through variable substrate recognition modules (28).
UPS & cancer
The UPS has been implicated in different diseases, including cancer. Deregulation of the UPS can contribute to oncopathogenesis through downregulation of cell-cycle and tumour-suppressor proteins (such as p53 and p27), or upregulation of oncogenic proteins (like NF-κB) (29,30). Remarkably, cancer cells are more susceptible to UPS targeting due to their increased metabolism and protein turnover. Thus, the UPS is of interest in cancer therapeutics, and, in fact, treatments already exist that are directed against this system. The proteasome inhibitor bortezomib was the first drug to be brought into clinical use that targets the UPS. It is a slowly reversible inhibitor of the 20S proteolytic core of the proteasome and serves as the first-line treatment in patients with multiple myeloma and with mantle-cell lymphoma (31). Development of drugs that target the different components of the UPS is ongoing.
UPS & GIST
The importance of the UPS in GIST is highlighted by its role under normal cellular conditions. Regulation of KIT activity is, in part, mediated by KIT ubiquitination that leads to receptor internalization and degradation (32). An important counter to KIT ubiquitination is heat shock protein 90 (HSP90), as it stabilises KIT, preventing its degradation (33). For this reason, GIST cell treatment with 17-allylamino-18-demethoxy-geldanamycin (17-AAG), which prevents HSP90 from stabilising client proteins, has been shown to significantly impair KIT activity regardless of KIT mutational status. Upon 17-AAG treatment, a significant reduction in phospho-KIT and total KIT expression was observed in imatinib-sensitive and imatinib-resistant cell lines, leading to decreased cell proliferation and survival was witnessed (34). However, despite substantial efforts have been made, the clinical development of HSP90 inhibition has been challenging, despite some evidences pointing out towards clinical benefit in these patients (35).
Additional support of the relevance of the UPS in GIST comes from Liu and colleagues (36) who found a relationship between the histone variant H2AX and GIST. H2AX is a major regulator of the DNA damage cellular response. Pertinent to GIST, DNA replication stress can cause excessive accumulation of soluble H2AX, leading to remarkable cellular toxicity (36,37). In the study, elevated levels of free, soluble histone variant H2AX were observed in GIST cells treated with imatinib, which sensitised the cells to apoptosis. On the other hand, untreated cells had downregulated H2AX levels due to PI3K- and mTOR-stimulated poly-ubiquitination that leads to H2AX proteasomal degradation (36). Therefore, H2AX was found to act as a tumour suppressor and the authors argue that inhibiting H2AX degradation with proteasomes inhibitors might serve as a novel GIST treatment. Indeed, building on these findings, they later tested the effects of the proteasome inhibitor bortezomib in GIST in one study and the effects of other proteasome inhibitors in a separate one (24,38). Treatment with bortezomib led to H2AX upregulation and, unexpectedly, to KIT transcriptional downregulation. The same results were seen with the additional proteasome inhibitors carfilzomib, ixazomib and delanzomib. Although the mechanism of action of bortezomib that causes KIT downregulation was not fully dissected, evidence was found that bortezomib induces a general transcriptional shutdown (38). This is likely thanks to the role of the UPS in the regulation of transcriptional activation.
Together, these results emphasise the biological relevance of the UPS in GIST and the potential of targeting the UPS, such as with proteasome inhibitors. Further research, however, is needed to identify novel targets of the UPS machinery pertinent to GIST biology. These types of treatments are particularly appealing as they could be used to treat GIST patients regardless of their KIT mutational status. Yet, treatments directed against the UPS are not the only manner that this system is relevant to GIST.
Targeted protein degradation: utilising the UPS against GIST
Using the cellular protein homeostasis system to our advantage in order to degrade proteins of interest has become possible via what is called ‘targeted protein degradation’. This involves the use of small molecules that, for example, degrade their target by hijacking the UPS. The primary examples of this are proteolysis targeting chimeras (PROTACs) and molecular glues.
PROTACS are heterobifunctional molecules that consist of two moieties, one binding the target protein and one an E3 ligase, connected by an appropriate linker. By being near each other, the E3 is able to induce poly-ubiquitination of the target protein and its consequent degradation by the proteasome. PROTACS tend to have a large-molecular-weight due to their modular design, which complicates their clinical usability (39). On the other hand, molecular glues are small-weight inducers of protein-protein interactions that do not have measurable binding affinity to their target protein (40). Thalidomide and its analogues (lenalidomide and pomalidomide), collectively known as immune-modulatory imide drugs (IMiDs), demonstrate the potential of molecular glues as therapeutics. They are used to treat different types of myeloma and function by inducing degradation of essential TFs. More specifically, their mechanism of action involves binding to cereblon (CRBN), a component of the CRBN-CRL4 ubiquitin ligase, and promoting interactions with the TFs IKZ1 and IKZ3, leading to their ubiquitination and subsequent proteasomal degradation (41).
Unlike PROTACs, which follow a rational drug design and thus makes their development more straightforward, discovery of molecular glues has so far been serendipitous. Yet, their peculiar molecular pharmacology means they can bind to targets deemed ‘undruggable’, such as TFs. Thus, generating rational approaches to develop novel molecular glues is of great importance.
Targeting of the TF ETV1 in GIST, which has been shown to be imperative for GIST biology, is therapeutically relevant. In fact, a clinical trial that aims on indirectly inhibiting ETV1 expression via combined imatinib/MEK162 treatment (a MEK inhibitor) is ongoing (42,43). Yet, specific targeting of ETV1 would be a desirable approach. Development of molecular glues (or PROTACs) against ETV1 could lead to a significant improvement in GIST treatments irrespective of KIT mutational status.
KIT degradation using targeted protein degradation would also be a potential therapeutic approach. Interestingly, KIT degradation appears to be mediated by the lysosome system rather than by the UPS (44). Because of this, developing lysosome-targeting chimeras (LYTACs), which are a novel protein degradation modality, would be of interest for GIST. Briefly, LYTACs function by recruiting a cell-surface lysosome-targeting receptor to a target membrane protein, resulting in their subsequent lysosomal degradation, thus following the same concept as PROTACs and molecular glues (45). Although still in early experimental phases, LYTACs have potential as cancer therapeutics, demonstrated by their success in ablating EGFR and HER2 receptors in hepatocellular carcinoma (HCC) cells (46). Hence, LYTACs directed against KIT, or PDGFRA, might constitute an important player in GIST therapy and should be developed.
Overall, therapies against the UPS machinery or that hijack the normal cellular mechanisms that regulate protein homeostasis represent novel mechanisms to treat GIST patients irrespective of their mutational status.
Tumour microenvironment and the immune system
The immune system is in charge of protection against pathogens and tumour formation. Immune responses are initiated upon recognition of foreign invaders, leading to a cascade of effector functions such as immune infiltration, antigen presentation by antigen-presenting cells (APCs), activation of natural killer (NK), T and B cells, and cytokine and chemokine secretion (47). Although cancers arise from normal cells, a high level of mutations trigger a phenotypical diversification to the extent that they may be recognised as foreign by the immune system (48). This recognition occurs via neoantigens expressed in tumours that are presented on major histocompatibility class I (MHCI) molecules to dendritic cells (DCs) and subsequently to T cells in secondary lymphoid organs (48). Upon priming, T cells specific for the tumour-antigen become activated, provided there is adequate co-stimulation, few regulatory T cells (Tregs) and no inhibitory stimuli (49). If this is successful, the activated effector T cells infiltrate the tumour and, upon recognition of the cancer’s neoantigens, initiate their effector functions to kill the cancer cells (49). However, tumours eventually adapt to overcome this immune surveillance.
Immune evasion by tumours can occur in different manners. For example, DCs and T cells may not recognise tumour antigens or would treat them as self-antigens, eliciting a tolerogenic response (i.e., T cell differentiation into Tregs). This can create an immunosuppressive microenvironment that prevents tumour infiltration by T cells (48,49). Suppression of immune responses by tumours occurs greatly by expression of inhibitory immune checkpoints—molecules that activate negative regulatory pathways (48). Although these checkpoints serve to maintain immune homeostasis and prevent damage to self-tissues, cancers can exploit these molecules for their advantage. Cytotoxic T-lymphocyte protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are the prime examples of inhibitory immune checkpoints (48). These receptors are expressed on the surface of activated T cells, but they inhibit anti-tumour responses in distinct manners. CTLA-4 prevents T cell proliferation in the initial phases of immune responses, while PD-1 inhibits T cells at later stages in peripheral tissues. The discovery of these immune checkpoint inhibitors has led to the development of immunotherapies against cancer, primarily employing anti-PD1, anti-PD-L1 and anti-CTLA4 drugs (50).
GIST and the immune system
The impact of the immune system in GIST is still not fully understood. However, recent studies have emerged that show how the immune system might be an important component of GIST. Tumour-infiltrating cells have been found in the disease microenvironment and they have been linked not only to disease outcomes, but also to the success of imatinib.
Imatinib has been shown to activate NK cells in mice and humans, resulting in NK cell-dependent anti-tumour effects (51). One study found that increased production of IFN-y by peripheral-blood NK cells can serve as prognostic factor for progression-free survival (PFS) of GIST patients treated with imatinib (52). The anti-tumour effects seen appear to have been mediated via interleukin killing of tumour cells rather than by direct NK cell killing, though the precise mechanism remains to be elucidated.
T cells have also been shown to be important players in GIST upon treatment with imatinib. Treatment with imatinib of KIT-mutant mice that developed spontaneous GIST led to increased number of activated CD8+ T cells, while promoting apoptosis of Treg cells within the tumour. This was attributed to a reduction in the expression of indoleamine 2,3-dioxygenase (IDO), a T cell inhibitor, by the tumour cells. Moreover, as the authors saw increased infiltrating levels of CD8+ T cells, they tested if combination therapy of imatinib with CTLA-4 blockade would result in enhanced anti-tumour efficacy. This was indeed the case and appeared to be mediated through an increase in the number of IFN-y producing CD8+ T cells (53). Based on these findings, a phase Ib study was designed to test the safety of combined KIT inhibition with CTLA-4 blockade. However, while safe, there was no significant benefit of this combinatorial treatment. Of relevance, the authors found evidence that IDO suppression can correlate with efficacy of GIST treatment (54). Yet, the conclusions from this study are limited due to the small cohort size and by the fact that most patients in the study had advanced, extensively pre-treated GIST. Thus, additional studies are warranted, ideally with early-stage GIST patients whose tumours are likely to be less heterogeneous and thus might respond better to this combinatorial therapy.
While the research mentioned above suggests an anti-tumour role of IFN-y secreted by immune cells, one study found evidence of a pro-tumorigenic role of this cytokine. Elevated levels of inhibitory receptors, especially those of PD-1, were found in tumour-infiltrating T cells of patients compared with T cells from matched blood. For this reason, the authors wanted to check the relevance of PD-L1 expression on GIST. Overall, they found a variable and heterogeneous expression of PD-L1. Using GIST cellular and murine models, they found that IFN-y increased the levels of PD-L1 on GIST cells, which was linked to STAT-1 activation by IFN-y. Interestingly, imatinib prevented this PD-L1 induction and combining imatinib with anti-PD-1 or anti-PD-L1 led to increased efficacy of imatinib in their mouse model (55). Another study that analysed the GIST microenvironment found elevated levels of CD4+ and CD8+ T cells, as well as M2 macrophages. They also found evidence that IFN-y can indeed induce PD-L1 expression in GIST cells (56). These contrasting results, with IFN-y appearing anti-oncogenic in some studies but pro-oncogenic in others, emphasise the intricacies of the immune system and the need to continue exploring its role in GIST.
As we have mentioned before, there is great heterogeneity between GIST patients. Thus, it is possible that the different roles observed of IFN-y, as well as the limited efficacy of the immunotherapies tested in GIST, are caused by this tumour diversity. This is indeed shown in one study that compared the immune profiles between KIT- and PDGFRA-driven GISTs, with the latter having a stronger immune signature. They argue that this enhanced immune infiltration may, at least partially, explain why patients with PDGFRA-driven GISTs have an overall better prognosis. Interestingly, they also saw that PDGFRA-mutants present a higher number of neoantigens. Because of this, they suggest that PDGFRA-mutant GISTs could respond better to immunotherapeutic approaches (57). Therefore, this study shows that the heterogeneity of GIST is not limited to the tumour, but that it expands to the immune microenvironment as well. Nonetheless, cancers are multifaceted diseases, having not only numerous mutations but also different interactions with their environment, implying that there are more factors that influence the immunogenic state of GISTs.
These studies demonstrate that the immune system plays an active role in GIST. The success of imatinib as a treatment for GIST appears to not only be caused by direct KIT/PDGFRA inhibition in GIST cells, but also through stimulation of the immune system in different manners. Although there are many indicators that GIST patients would therefore benefit from immunotherapies in combination with TKIs, particularly imatinib, the few clinical trials undertaken have shown little success. This might be due to the trials being performed in patients with advanced GISTs, but also because it could be that only specific subsets of patients benefit from these therapies. All the above underscore the complexity of the immune system in GIST, as is the case with other cancers. Notably, the studies done so far have mostly classified different GIST immune profiles, but not much detail is known about the interplay between the immune microenvironment and GIST. Therefore, we need to improve our understanding of the immune system in GIST in order to develop therapeutic strategies that make use of its power. Yet, the presence of an active immune system suggests that targeting this system can result in potent therapeutic gains for GIST patients, especially when biomarkers are identified for those who could respond the most to immunotherapies.
GIST and the cancer-associated fibroblasts (CAFs)
The immune system is not the only aspect of the tumour microenvironment that is important. CAFs, which represent the most abundant cells in the tumour microenvironment, have been shown to promote tumour progression and drug resistance. A recent study found that gastric fibroblasts turn into CAFs via TGF-B1 secreted from GIST cells. This created a positive feedback loop by which CAFs then secreted additional TGF-B1, further increasing CAF levels. Moreover, TGF-B1 was found to promote GIST cell migration, which was abrogated upon anti-TGF-B1 antibodies (58). Overall, this study demonstrates that GIST cells interact with their microenvironment and that TGF-B1 might be an important player in GIST metastasis. Therefore, it highlights how the interplay between GIST and its microenvironment needs further exploration, as it can reveal novel therapeutic targets—in this case, TGF-B1 inhibition to target metastasis.
Other recent approaches
Preventing therapeutic adaptation to KIT/PDGFRA targeted inhibition
As mentioned throughout this review, GIST therapies have focused on targeting its high reliance—and thus vulnerability—on KIT/PDGFRA oncogenic signalling. Yet, this selective pressure leads to the positive selection and expansion of clones harbouring secondary mutations, resulting in drug resistance. Yet, some mechanisms of therapeutic adaptation (ka adaptive resistance) have been described and appear to aid in GIST cell survival, first, facilitating the emergence of true resistance mechanisms later. For instance, it has been shown that fibroblast growth factor receptor (FGFR) activation upon therapeutic KIT inhibition diminishes the effects of imatinib by reactivating the MAPK pathway. Combined inhibition of KIT and FGFR was synergistic in suppressing GIST growth in preclinical models (59). It was thus hypothesised that combined inhibition of both receptors may suppress GIST growth while preventing drug resistance. This provided the basis for a phase I study investigating the combination of imatinib with BGJ398 (an FGFR inhibitor). Unfortunately, the trial reported significant toxicity and had to be prematurely stopped. Interestingly, 25% of patients experienced prolonged stabilisation of the disease, suggesting that this might be a successful approach if the toxicity of the treatment can be ameliorated (60).
Another compensatory mechanism that has been described upon KIT/PDGFRA inhibition is the expression of the MET oncogene. MET expression was found to be induced by treatment with imatinib, resulting, likely, in a ‘kinase switch’. Inhibition of MET with crizotinib or with cabozantinib resulted in increased GIST apoptosis, suggesting that MET is an important therapeutic target (61). To test this rationale, a phase II trial with cabozantinib was performed. To justify the further exploration of cabozantinib, a minimum of 21 of the first 42 patients had to be progression-free at week 12. A total of 24 were found to be progression-free at week 12, thus supporting the preclinical evidence that MET inhibition is relevant clinically (62). Therefore, a larger clinical study should be undertaken to further explore the efficacy of cabozantinib/MET inhibition in GIST.
While the studies above have shown mixed results, it is worth noticing that they were performed on patients with advanced GISTs. Therefore, these regimes may have underperformed because of the patients existing resistance to imatinib. Consequently, it would be relevant to test these novel approaches in previously non-treated GISTs. They might prove more successful and help prevent, or at least significantly delay, the onset of secondary mutations and thus the need for alternative/2nd–4th lines of treatments.
We recently tested preclinically a combinatorial treatment of ripretinib and MAPK inhibition, akin to the study that combined with MAPK inhibitors to suppress ETV1 and maximise treatment response. Combination of ripretinb with MEKi showed superior efficacy than imatinib in different cellular models, particularly in imatinib-resistant cell lines (63). As ripretinib has a broader KIT/PDGFRA spectrum that it can target compared to imatinib, combination of ripretinib, rather than imatinib, with MEK inhibitors could be more clinically relevant if successful in clinical trials. Likewise, we have recently identified the E3 ubiquitin ligase FBXO32 (Atrogin-1) as a critical mediator of therapeutic adaptation to KIT/PDGFRA-targeted agents. Remarkably, blockage of the ubiquitin ligase cascade with UAE inhibitor TAK-243 prevents treatment adaptation and substantially induces cell death (64).
Using G protein-coupled receptor 20 (GPR20) as Trojan horse to target GIST
Therapeutic approaches against GIST that are independent of their mutational status will result in significant progress in GIST management. With this in mind, Iida et al. found GPR20 to be almost ubiquitously expressed in GIST apparently irrespective of their mutational driver (e.g., KIT/PDGFRA driven, NF1-related, SDH-deficient, etc.). For this reason, they produced DS-6157a, an anti-GPR20 antibody-drug conjugate with a tetrapeptide-based linker and DNA topoisomerase I inhibitor exatecan-derivative (DXd). They tested this drug in xenograft models and found high anti-tumour activity in GIST, including in models resistant to imatinib, sunitinib and regorafenib. As expected, efficacy of DS-6157a was dependent on GPR20 expression. Moreover, favourable pharmacokinetics and safety profiles were noted pre-clinically, supporting the further development of this drug (65). Interestingly, despite the prominence of GPR20 expression in GIST cells, its function is yet to be elucidated. These findings are especially promising as GPR20 is expressed in different GISTs regardless of their molecular classification, thus this approach holds great potential to treat most—if not all—GIST patients.
Concluding remarks
Management of GIST has improved dramatically upon imatinib approval. This success led to the development of additional KIT/PDGFRA inhibitors, resulting in a significant improvement in the survival of GIST patients. However, most patients develop resistance to imatinib and eventually become resistant to all further approved treatments. For this reason, novel therapeutics are needed, especially those that can circumvent this resistance. Additionally, treatments are needed that can target GISTs no matter their mutational driver. One approach being studied is the use of combinatorial treatments to overcome adaptive resistance. Still more experimental, but with promise, is the targeting of GIST via the ubiquitously expressed GPR20. We hereby also discussed possible alternative directions in the treatment of GIST such as targeting of the UPS (or hijacking it), for example through development of HSP90 inhibitors or ETV1 degraders. Additionally, targeting the tumour microenvironment to enhance immune responses or to prevent metastasis are potential treatment options that could greatly benefit GIST patients. All of these approaches, however, merit further investigation before they become a clinical reality. Likewise, the great majority of these novel perspectives apply to KIT/PDGFRA-mutant GIST, and therefore, further research should be done in other molecular subtypes. Therefore, the Achilles heel of GIST is multifaceted and will require the development of diverse approaches that can target this disease from different angles.
Acknowledgments
Funding: This project was funded in part by an ISCIII PI19/01271, and co-funded by FEDER; Asociación Española Contra el Cáncer (AECC)—CLSEN20004SERR; 1st triannual grant from the Fundación Mari Paz Jiménez Casado; and FERO Foundation to CS.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Claudia Valverde, Nadia Hindi) for the series “Management of Gastrointestinal Stromal Tumors” published in Gastrointestinal Stromal Tumor. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://gist.amegroups.com/article/view/10.21037/gist-21-11/rc
Peer Review File: Available at https://gist.amegroups.com/article/view/10.21037/gist-21-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gist.amegroups.com/article/view/10.21037/gist-21-11/coif). The series “Management of Gastrointestinal Stromal Tumors” was commissioned by the editorial office without any funding or sponsorship. CS has received research funding (institution) from Karyopharm, Pfizer Inc., Deciphera Pharmaceuticals, and Bayer AG; consulting fees (advisory role) from CogentBio, Immunicum AB, Deciphera Pharmaceuticals and Blueprint Medicines; payment for lectures from Bayer AG and Blueprint Medicines; and travel grants from PharmaMar, Pfizer, Bayer AG, Novartis and Lilly. The authors have no other conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ducimetière F, Lurkin A, Ranchère-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011;6:e20294. [Crossref] [PubMed]
- Sommer G, Agosti V, Ehlers I, et al. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci U S A 2003;100:6706-11. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [Crossref] [PubMed]
- Mol CD, Dougan DR, Schneider TR, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem 2004;279:31655-63. [Crossref] [PubMed]
- Yuzawa S, Opatowsky Y, Zhang Z, et al. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell 2007;130:323-34. [Crossref] [PubMed]
- Lasota J, Corless CL, Heinrich MC, et al. Clinicopathologic profile of gastrointestinal stromal tumors (GISTs) with primary KIT exon 13 or exon 17 mutations: a multicenter study on 54 cases. Mod Pathol 2008;21:476-84. [Crossref] [PubMed]
- Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 2003;125:660-7. [Crossref] [PubMed]
- Bosbach B, Rossi F, Yozgat Y, et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc Natl Acad Sci U S A 2017;114:E8448-57. [Crossref] [PubMed]
- Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001;61:8118-21. [PubMed]
- Duensing A, Medeiros F, McConarty B, et al. Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs). Oncogene 2004;23:3999-4006. [Crossref] [PubMed]
- Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage-specific survival factor in GIST and cooperates with KIT in oncogenesis. Nature 2010;467:849-53. [Crossref] [PubMed]
- Serrano C, Wang Y, Mariño-Enríquez A, et al. KRAS and KIT Gatekeeper Mutations Confer Polyclonal Primary Imatinib Resistance in GI Stromal Tumors: Relevance of Concomitant Phosphatidylinositol 3-Kinase/AKT Dysregulation. J Clin Oncol 2015;33:e93-6. [Crossref] [PubMed]
- Bauer S, Duensing A, Demetri GD, et al. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene 2007;26:7560-8. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Serrano C, Fletcher JA. Overcoming heterogenity in imatinib-resistant gastrointestinal stromal tumor. Oncotarget 2019;10:6286-7. [Crossref] [PubMed]
- Serrano C, George S, Valverde C, et al. Novel Insights into the Treatment of Imatinib-Resistant Gastrointestinal Stromal Tumors. Target Oncol 2017;12:277-88. [Crossref] [PubMed]
- Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64-74. [Crossref] [PubMed]
- Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol 2005;23:5357-64. [Crossref] [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 2011;11:865-78. [Crossref] [PubMed]
- Shen M, Schmitt S, Buac D, et al. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin Ther Targets 2013;17:1091-108. [Crossref] [PubMed]
- Rausch JL, Ali AA, Lee DM, et al. Differential antitumor activity of compounds targeting the ubiquitin-proteasome machinery in gastrointestinal stromal tumor (GIST) cells. Sci Rep 2020;10:5178. [Crossref] [PubMed]
- Park J, Cho J, Song EJ. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res 2020;43:1144-61. [Crossref] [PubMed]
- Gupta I, Singh K, Varshney NK, et al. Delineating Crosstalk Mechanisms of the Ubiquitin Proteasome System That Regulate Apoptosis. Front Cell Dev Biol 2018;6:11. [Crossref] [PubMed]
- Lorick KL, Jensen JP, Fang S, et al. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc Natl Acad Sci U S A 1999;96:11364-9. [Crossref] [PubMed]
- Lydeard JR, Schulman BA, Harper JW. Building and remodelling Cullin-RING E3 ubiquitin ligases. EMBO Rep 2013;14:1050-61. [Crossref] [PubMed]
- Orlowski RZ, Baldwin AS Jr. NF-κB as a therapeutic target in cancer. Trends Mol Med 2002;8:385-9. [Crossref] [PubMed]
- Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle 2010;9:2342-52. [Crossref] [PubMed]
- Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol 2017;14:417-33. [Crossref] [PubMed]
- Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev 2012;92:1619-49. [Crossref] [PubMed]
- Fumo G, Akin C, Metcalfe DD, et al. 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is effective in down-regulating mutated, constitutively activated KIT protein in human mast cells. Blood 2004;103:1078-84. [Crossref] [PubMed]
- Bauer S, Yu LK, Demetri GD, et al. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res 2006;66:9153-61. [Crossref] [PubMed]
- Shimomura A, Yamamoto N, Kondo S, et al. First-in-Human Phase I Study of an Oral HSP90 Inhibitor, TAS-116, in Patients with Advanced Solid Tumors. Mol Cancer Ther 2019;18:531-40. [Crossref] [PubMed]
- Liu Y, Tseng M, Perdreau SA, et al. Histone H2AX is a mediator of gastrointestinal stromal tumor cell apoptosis following treatment with imatinib mesylate. Cancer Res 2007;67:2685-92. [Crossref] [PubMed]
- Gunjan A, Verreault A A. Rad53 kinase-dependent surveillance mechanism that regulates histone protein levels in S. cerevisiae. Cell 2003;115:537-49. [Crossref] [PubMed]
- Bauer S, Parry JA, Mühlenberg T, et al. Proapoptotic activity of bortezomib in gastrointestinal stromal tumor cells. Cancer Res 2010;70:150-9. [Crossref] [PubMed]
- Kannt A, Đikić I. Expanding the arsenal of E3 ubiquitin ligases for proximity-induced protein degradation. Cell Chem Biol 2021;28:1014-31. [Crossref] [PubMed]
- Mayor-Ruiz C, Bauer S, Brand M, et al. Rational discovery of molecular glue degraders via scalable chemical profiling. Nat Chem Biol 2020;16:1199-207. [Crossref] [PubMed]
- Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014;343:301-5. [Crossref] [PubMed]
- Ran L, Sirota I, Cao Z, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov 2015;5:304-15. [Crossref] [PubMed]
- Memorial Sloan Kettering Cancer Center. A Phase Ib/II Study of MEK162 in Combination With Imatinib Mesylate in Patients With Untreated Advanced Gastrointestinal Stromal Tumor (GIST). 2021. [cited 2021 May 20]. Available online: https://clinicaltrials.gov/ct2/show/NCT01991379
- Masson K, Heiss E, Band H, et al. Direct binding of Cbl to Tyr568 and Tyr936 of the stem cell factor receptor/c-Kit is required for ligand-induced ubiquitination, internalization and degradation. Biochem J 2006;399:59-67. [Crossref] [PubMed]
- Banik SM, Pedram K, Wisnovsky S, et al. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020;584:291-7. [Crossref] [PubMed]
- Ahn G, Banik SM, Miller CL, et al. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat Chem Biol 2021;17:937-46. [Crossref] [PubMed]
- Haynes BF, Soderberg KA, Fauci AS. Introduction to the Immune System. In: Jameson JL, Fauci AS, Kasper DL, et al. editors. Harrison’s Principles of Internal Medicine. 20th ed. New York: McGraw-Hill Education, 2018.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Borg C, Terme M, Taïeb J, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 2004;114:379-88. [Crossref] [PubMed]
- Ménard C, Blay JY, Borg C, et al. Natural killer cell IFN-gamma levels predict long-term survival with imatinib mesylate therapy in gastrointestinal stromal tumor-bearing patients. Cancer Res 2009;69:3563-9. [Crossref] [PubMed]
- Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 2011;17:1094-100. [Crossref] [PubMed]
- D'Angelo SP, Shoushtari AN, Keohan ML, et al. Combined KIT and CTLA-4 Blockade in Patients with Refractory GIST and Other Advanced Sarcomas: A Phase Ib Study of Dasatinib plus Ipilimumab. Clin Cancer Res 2017;23:2972-80. [Crossref] [PubMed]
- Seifert AM, Zeng S, Zhang JQ, et al. PD-1/PD-L1 Blockade Enhances T-cell Activity and Antitumor Efficacy of Imatinib in Gastrointestinal Stromal Tumors. Clin Cancer Res 2017;23:454-65. [Crossref] [PubMed]
- Pantaleo MA, Tarantino G, Agostinelli C, et al. Immune microenvironment profiling of gastrointestinal stromal tumors (GIST) shows gene expression patterns associated to immune checkpoint inhibitors response. Oncoimmunology 2019;8:e1617588. [Crossref] [PubMed]
- Vitiello GA, Bowler TG, Liu M, et al. Differential immune profiles distinguish the mutational subtypes of gastrointestinal stromal tumor. J Clin Invest 2019;129:1863-77. [Crossref] [PubMed]
- Yoon H, Tang CM, Banerjee S, et al. TGF-β1-mediated transition of resident fibroblasts to cancer-associated fibroblasts promotes cancer metastasis in gastrointestinal stromal tumor. Oncogenesis 2021;10:13. [Crossref] [PubMed]
- Li F, Huynh H, Li X, et al. FGFR-Mediated Reactivation of MAPK Signaling Attenuates Antitumor Effects of Imatinib in Gastrointestinal Stromal Tumors. Cancer Discov 2015;5:438-51. [Crossref] [PubMed]
- Kelly CM, Shoushtari AN, Qin LX, et al. A phase Ib study of BGJ398, a pan-FGFR kinase inhibitor in combination with imatinib in patients with advanced gastrointestinal stromal tumor. Invest New Drugs 2019;37:282-90. [Crossref] [PubMed]
- Cohen NA, Zeng S, Seifert AM, et al. Pharmacological Inhibition of KIT Activates MET Signaling in Gastrointestinal Stromal Tumors. Cancer Res 2015;75:2061-70. [Crossref] [PubMed]
- Schöffski P, Mir O, Kasper B, et al. Activity and safety of the multi-target tyrosine kinase inhibitor cabozantinib in patients with metastatic gastrointestinal stromal tumour after treatment with imatinib and sunitinib: European Organisation for Research and Treatment of Cancer phase II trial 1317 'CaboGIST'. Eur J Cancer 2020;134:62-74. [Crossref] [PubMed]
- Gupta A, Singh J, García-Valverde A, et al. Ripretinib and MEK Inhibitors Synergize to Induce Apoptosis in Preclinical Models of GIST and Systemic Mastocytosis. Mol Cancer Ther 2021;20:1234-45. [Crossref] [PubMed]
- García-Valverde A, Rosell J, Sayols S, et al. E3 ubiquitin ligase Atrogin-1 mediates adaptive resistance to KIT-targeted inhibition in gastrointestinal stromal tumor. Oncogene 2021;40:6614-26. [Crossref] [PubMed]
- Iida K, Abdelhamid Ahmed AH, et al. Identification and Therapeutic Targeting of GPR20, Selectively Expressed in Gastrointestinal Stromal Tumors, with DS-6157a, a First-in-Class Antibody-Drug Conjugate. Cancer Discov 2021;11:1508-23. [Crossref] [PubMed]
Cite this article as: Olivares-Rivas I, García-Valverde A, Serrano C. Which is the Achilles heel of gastrointestinal stromal tumour and how to use it for the future?—a review of novel therapeutic paradigms. Gastrointest Stromal Tumor 2022;5:3.


